 News Release
News ReleasePathAI Launches ArtifactDetect Model on AISight, Pioneering Automated Slide Quality Analysis in Pathology Labs
PathAI
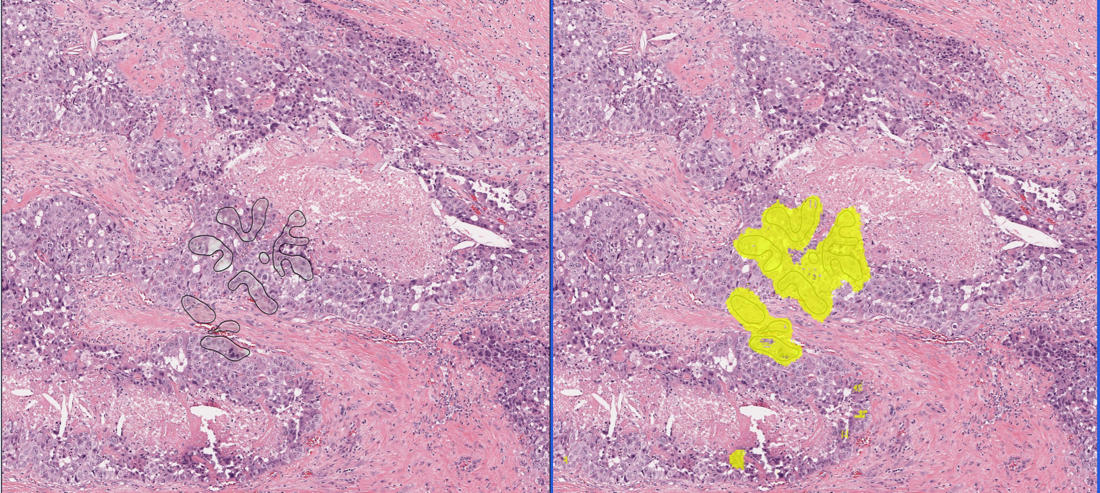
PathAI, a leading digital and computational pathology company which provides precision pathology solutions has announced the availability of ArtifactDetect 1 on AISight ™ 2, PathAI’s digital pathology image management system. This product automates the detection of artifacts on digital pathology whole slide images and quantifies the extent of artifact on any WSI. Artifacts are unintentional morphological features that do not have any histopathological relevance. Artifacts can limit the utility of these images for review by pathologists and require replacement of the image, which can create delays in slide review and case turnaround time. The addition of ArtifactDetect to the menu of algorithms available through AISight broadens the set of efficiencies that are enabled through computational pathology and improved laboratory operations with a digital workflow. This technology automatically identifies poor-quality samples, allowing for timely interventions such as re-staining or re-scanning, thereby saving valuable resources on scanning tech, histotechnology, and pathologist time. This announcement addresses a crucial gap in pathology labs — the lack of a standardized slide quality control system. Pathologists often spend significant time reviewing non-evaluable slides due to the absence of established quality standards. With ArtifactDetect, PathAI aims to provide quality control and workflow optimization features, empowering pathology labs worldwide. “We are very excited to partner with PathAI to enhance automation and accuracy in our laboratories, benefiting both our operations and the value we bring to our beneficiaries,” remarked Dr. Fernando Soares, Full Professor at the University of Sao Paolo and Head of the Department of Anatomic Pathology at Rede D’Or. Rede D’Or is the largest integrated health network in Brazil with more than 70 hospitals and 55 oncology clinics in operation across more than ten states. “We have been testing the AIM-HER2 Breast Algorithm on our diverse cohorts to much success and are excited to expand our collaboration with PathAI to include algorithms that can drive workflow efficiencies and help us better track the quality of our whole slide images.” "The absence of a consistent slide quality standard leads to inefficiencies and challenges in pathology labs," says Dr. Eric Walk, Chief Medical Officer at PathAI. "Our goal is to distribute our advanced algorithms to foster a standardized approach, significantly reducing the time spent on non-evaluable slides." To learn more about AISight, PathAI’s Image Management System, ArtifactDetect or any of PathAI’s other algorithm product solutions, please visit https://www.pathai.com/ap-lab-solutions/ or request a meeting by contacting digitaldx@pathai.com. 1,2 ArtifactDetect and AISight are For Research Use Only. Not for use in diagnostic procedures. About PathAI PathAI is the only AI-focused technology company to provide comprehensive precision pathology solutions for clinical trials and laboratory use. Rigorously trained and validated with data from more than 15 million annotations, its AI-powered models can be leveraged to optimize the analysis of pathology samples to improve efficiency and accuracy of pathology interpretation, as well as to better gauge therapeutic efficacy and accelerate drug development for complex diseases. About AISight AISight, introduced earlier this year by PathAI, is a comprehensive digital pathology image management system, currently offered for Research Use Only. AISight provides best-in-class image and case management, ingestion, and viewing while seamlessly enabling access and deployment of AI applications. AISight can also be integrated bidirectionally with laboratory information systems for streamlined workflow adoption. Anatomic pathology laboratories of all sizes and specialties – including health systems, reference laboratories, independent pathology labs, and academic medical centers – may utilize AISight. Contact Details SVM Public Relations and Marketing Communications Maggie Naples +1 401-490-9700 pathai@svmpr.com Company Website https://www.pathai.com/
November 30, 2023 10:00 AM Eastern Standard Time
Image