 News Release
News Release
Livespo Navax – World’s first spore probiotics in nasal spray form triumphs in ongoing clinical trials tackling influenza-related respiratory inflammation
LiveSpo
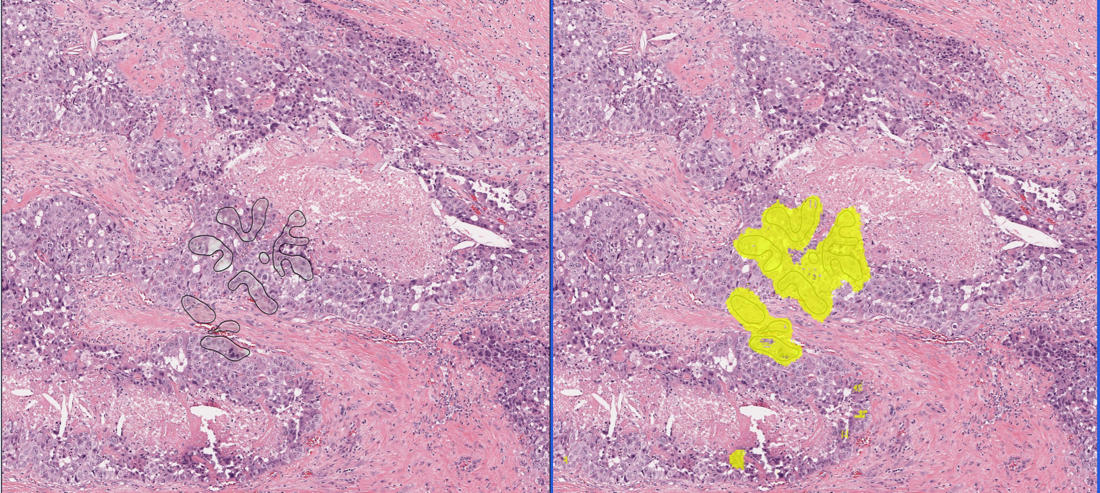
HANOI, VIETNAM - Media OutReach - 30 November 2023 - A recent publication in the Scientific Reports - Nature journal has once again highlighted the clinical trials of LiveSpo Navax spore probiotic spray. These trials focus on its effectiveness against respiratory tract inflammation from the influenza virus. This news marks a significant milestone for the Vietnamese medical sector, especially in setting a trend in global research and application of spore probiotics for respiratory well-being. Using a nasal spray with Bacillus probiotics in treating influenza patients has significantly reduced the total treatment time by up to 60 per cent (from five days for the control group to just two days for the spore probiotics spray group). Additionally, it increased treatment efficacy by 58 per cent, aiding in the complete recovery from symptoms such as runny nose, fever, sneezing, cough, rapid heartbeat, and rapid breathing. The decline in clinical symptoms corresponds with a drop in both viral and bacterial counts, notably decreasing by approximately 400 and 1,000 times on the second treatment day compared to the initial day of hospitalisation. The clinical trials confirm that the nasal spray containing Bacillus probiotics reduces treatment duration, lowering the chances of bacterial infection or severe respiratory tract complications. This not only helps cut treatment costs but also reduces antibiotic consumption for infections, fostering improved patient health. This positive signal is crucial for the medical community as it contributes to easing the burden on hospitals during disease outbreaks. Furthermore, the clinical trials indicate that the Bacillus probiotics, when formulated as a spray, offer superior effectiveness through interaction with the mucosal immune system of the nasal cavity. This means that the spore probiotics nasal spray not only supports the treatment of influenza-infected patients but also aids in treating respiratory tract inflammation caused by other viruses such as RSV, adenovirus, rhinovirus, coronavirus, etc. Regular use of Bacillus probiotics spray proves beneficial in enhancing the respiratory barrier, thereby boosting the effectiveness of virus-prevention vaccines. Probiotics, also known as a type of medicinal probiotics, serve as an effective solution in alleviating symptoms of respiratory diseases. In a previous study published in the Scientific Reports - Nature journal in July 2022, LiveSpo Navax supported the treatment of respiratory syncytial virus (RSV) in children. It significantly reduced the treatment time by more than one day and lowered the RSV concentration by over 600 times, surpassing the effectiveness of using physiological saline. This scientific research ranked among the top 100 downloaded microbiology articles in 2022 in the Nature Journal, demonstrating the widespread interest from scientists and readers worldwide and contributing significant values to the community. The two internationally validated clinical studies have confirmed that the LiveSpo Navax nasal spray, containing Bacillus probiotics, has demonstrated no side effects, guaranteeing complete safety for children. All patients using LiveSpo Navax in conjunction with standard treatment medications showed no signs of local bacterial infection or any digestive issues like vomiting, diarrhoea, or other abnormal symptoms. Therefore, in the future, this product will be fully suitable for inclusion in treatment plans for patients infected with respiratory viruses or bacteria. Dr. Nguyen Hoa Anh, Director of the Spore Probiotics Research Center shared: "We are proud to offer an effective support solution for patients with respiratory infections caused by viruses, bacteria, or acute inflammation. Moving forward, we will continue to research, develop, and bring the LiveSpo Navax product to consumers in Viet Nam and globally." With groundbreaking technology in spore probiotics - medicinal probiotics, LiveSpo Pharma aims to build a future free of antibiotics for everyone. Dang Quoc Hung, CEO of LiveSpo Pharma, stated: "At LiveSpo, we always strive to deliver the best products to our customers and the community. With the expertise of our scientific team, who have achieved notable research milestones in microbiology domestically and internationally, we are confident in developing and manufacturing effective and convenient spore probiotic products without side effects. This effort contributes to reducing antibiotic use and minimising antibiotic resistance in Viet Nam and the world. This is also LiveSpo's mission for development." As confirmed by the two successful publications in the prestigious Scientific Reports - Nature journal, LiveSpo has asserted its breakthrough in the products resulting from the company's extensive research and development efforts. LiveSpo takes pride in introducing Viet Nam's spore probiotics technology to the markets of developed countries which spearhead global medical advancements. This groundwork sets the stage for the future growth of Vietnamese enterprises, emphasising the expansion into global markets and the international promotion of Vietnamese brands and products. With a focus on product development, LiveSpo aims to create value for the community. The company's successful development of the Bacillus probiotics spray, which effectively aids respiratory disease treatment and minimises the risk of severe inflammation and prolonged antibiotic use, is helping pave the way toward a future less reliant on antibiotics. With its nature as a type of medical probiotic, LiveSpo products will help strengthen the natural immune barrier for the body when used over an extended period, thereby contributing to a community immune system if widely applied. This serves as a natural shield, protecting the community from diseases caused by viruses and bacteria. About LiveSpo Pharma LiveSpo is an R&D, manufacturing, and distribution brand of Spobiotics (probiotics in spore form) for digestive and respiratory health, leading in breakthrough technology, rapid effectiveness, and convenience, aiming for "A Future Without Antibiotics". Hotline: 1800.088808 Website: livespo.com Contact Details Livespo Media Contact (Mr) Chu Minh Dac +84 98 459 95 96 dac.cm@livespo.com Company Website https://livespo.com/
November 30, 2023 08:55 AM Eastern Standard Time
Image
 News Release
News Release