News Release
News Release
MetroPlusHealth Brings Behavioral Health Services In-house, Citing Need for Integrated Care of Body and Mind
MetroPlusHealth
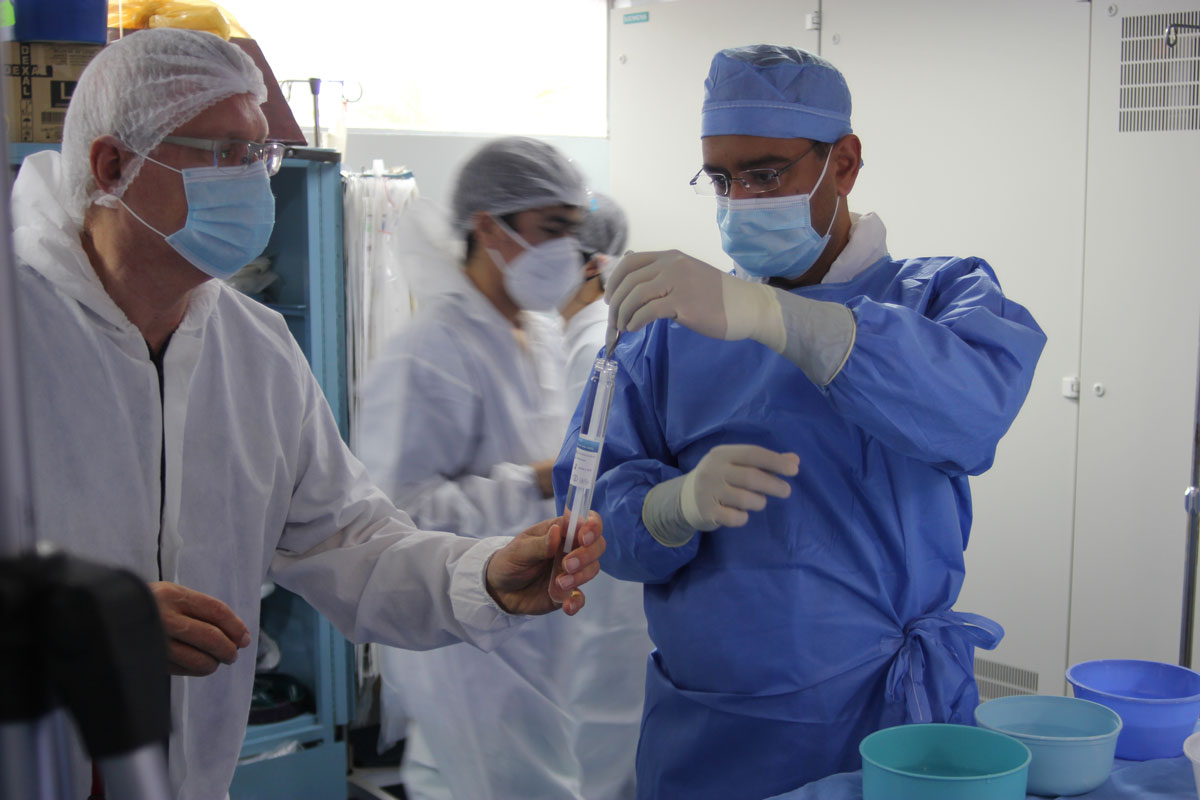
MetroPlusHealth, New York City’s five-star health plan, announced today the successful completion of its year-long effort to bring behavioral health (BH) services in-house. Now, MetroPlusHealth staff, part of the largest BH provider in New York State – NYC Health + Hospitals – will be providing both BH and medical benefits to its more than 625,000 members. As New York City continues to recover from the COVID-19 pandemic, the integration of body and mind issues and coordinated care of both have become more important than ever before. Amidst an uptick in the utilization of BH services by its members, including a bump of nearly 85 % in BH telehealth services, a recent survey* conducted by MetroPlusHealth found that over 55% of New Yorkers believe the pandemic has negatively impacted their mental health. The transition to in-house BH services will help address the surge in BH-related issues, offering MetroPlusHealth members a more effective, holistic care experience, and will result in improved medical and mental health outcomes for members and greater efficiency for the health plan. The Plan’s approach to integrated care has already garnered significant success, earning national recognition for its innovation and, in New York State, the #1 ranking in quality. Over a nearly forty-year span, MetroPlusHealth has grown from a tiny pilot program working out of Manhattan’s Metropolitan Hospital to a major health insurer, covering hundreds of thousands of lives across the five boroughs. Delivering BH services to its members is the latest forward step in the steady evolution of one of New York City’s largest health plans. “Behavioral health benefits must be an integral component of our health plans. These services enable us to offer our members the full range of coordinated care,” said Dr. Talya Schwartz, President and CEO of MetroPlusHealth. “Our mission, as a health plan, embraces taking care of the complete person. When we care for the body, we can’t just ignore the mind. New York City has always been demanding, and its residents face many stresses. But COVID-19 has placed new pressures on the health care system, on New Yorkers, and our most vulnerable members, as well. As New York City’s homegrown health plan and proud New Yorkers ourselves, we quickly realized that these times require innovative thinking and approaches. Bringing BH in-house was one of the most critical initiatives we launched during the pandemic. Our members need our full attention to their care, and that includes their behavioral health.” MetroPlusHealth is committed to treating physical health and behavioral health as equally important to its members' wellbeing. Traditionally, these concerns have been managed separately by most health plans, with BH outsourced to vendors. Over the past eighteen months, MetroPlusHealth has done significant work to integrate a behavioral health model of care, which aims to heighten staff awareness and synergy to ensure that members’ physical and mental health issues are addressed together to improve care quality and efficiency. Under this new model of care, MetroPlusHealth now enlists and collaborates with its network of primary care physicians (PCPs) to identify behavioral health issues at the front lines. This will result in PCPs becoming more acutely aware of their patient’s behavioral health challenges and effectively starting recovery as soon as possible. Dr. Schwartz emphasized the advantages the new BH arrangement affords MetroPlusHealth’s participating providers. “We believe our new BH benefit management is less restrictive and more collaborative with our in-network providers. Less the stick approach with utilization management and more aligned with our providers through VBP contracting. This difference is not trivial, as credit for savings has to be split between physical health and BH, but our new BH model is more likely to be successful than when we outsourced behavioral health. Overall, the transition to in-house BH services is great for members, providers, and us. Definitely a win-win-win.” MetroPlusHealth’s decision to transition its behavioral health to in-house management “unlocks a new universe of quality and efficiency opportunities that would have been difficult to achieve with outside partners,” said Dr. Sanjiv Shah, Chief Medical Officer of MetroPlusHealth. “Having mental health services in-house puts mental and medical health on equal footing and importance. BH impacts physical health, and physical health impacts BH. We know that people with BH issues have significantly worse outcomes and shorter life spans. After cardiac events, patients frequently experience depression and anxiety and addressing those conditions may lead to better cardiac outcomes.” With in-house services, MetroPlusHealth’s BH experts can now screen for complications in real-time before conditions worsen, eliminating the issue of not having access to care because of insurance. They can also supplement medical treatment by addressing social determinants of health such as housing, food insecurity, and employment. “The new BH model,” said Dr. Shah, “will make it much easier for us to identify, monitor, and partner with our members in the successful treatment of their physical and mental health needs, and their social determinants, as well.” “Our team works closely with some of the Plan’s most at-risk members,” stated Anna Reyna, LCSW, CCM, Manager, ICM (Integrated Care Management) Task Force at MetroPlusHealth. “It’s so exciting to now have an in-house behavioral health department that can support our work with high risk, homeless members, who often have significant mental health and substance use disorders, alongside complex medical conditions. These members may need assistance with so many aspects of their care that it can be overwhelming and discouraging to people who are already struggling. An in-house behavioral health department that understands the challenges of our most vulnerable members, and can assist with their stabilization, is a tremendous advantage to our members.” MetroPlusHealth’s integrated BH program is designed to: Ensure access to a broad range of services like Home & Community-Based Services (HCBS), which support the acquisition of life skills, job training, employment support, social skills, and family therapy. Support Behavioral Health Children and Family Treatment and Support Services (CFTSS), an array of therapy, rehabilitation, and psychosocial services for children and youth under age 21. Create tailored partnerships for extremely vulnerable (e.g., formerly incarcerated and homeless), ensuring strong alignment/coordination with City agencies, H+H, and CBOs. Provide individualized and comprehensive care plans designed for each member, considering BH, Medical and Social Determinants of Health. Person-Centered Service Plan (PCSP) will emphasize shared decision-making approaches that empower members, provide choice and minimize stigma. Actively communicate with the member’s other care coordinators and providers to ensure health and wellness goals are met. Care coordination activities will be the foundation for care plans and foster individual responsibility for health awareness. Work with the provider community to provide member-specific feedback. MetroPlusHealth’s BH program and patient outcomes will: Provide a whole-person approach to treatment, prevention and wellness, including a focus on addressing social determinants. Reduce avoidable inpatient and emergency department utilization by ensuring effective care management, utilization management and access to quality providers. Promote recovery from substance use disorder through effective connection to Medication-Assisted Treatment (MAT) and/or outpatient treatment. Develop operational agreements with the provider community to align on effective rendering of evidence-based treatments. Besides the robust network of BH providers now available to them through their health plan, MetroPlusHealth members can also utilize MetroPlus Virtual Visit. This no-cost 24/7 telehealth program connects them to board-certified therapists and psychiatrists by smartphone, tablet, or computer. With COVID-19, it is essential that members always have access to telehealth. Virtual Visits enable members to get help when they need it, free of stigma and free of charge. Much of the mechanics of the BH transition to an in-house model may be clinical, Dr. Talya Schwartz pointed out. “But, ultimately, this transition is about ensuring that hundreds of thousands of New Yorkers, our members, our neighbors, get seamless access to the mental health services that now, more than ever, they need.” ### About MetroPlusHealth Since 1985, MetroPlus Health Plan has built a reputation for providing access to affordable, quality health care to residents across New York City. MetroPlusHealth is the plan of choice for over 625,000 New Yorkers and has a five-star rating based on the State’s 2020 Consumer’s Guide to Medicaid and Child Health Plus Managed Care Plans in New York City. The health plan’s robust network of primary care doctors and specialists includes many independent community providers. Culturally sensitive, and fluent in more than 40 languages, MetroPlusHealth’s staff is as diverse as the great city it serves. For more information about MetroPlusHealth plans, benefits, and services, visit www.metroplus.org and join the conversation at facebook.com/metroplushealth and Twitter @metroplushealth. MetroPlusHealth is part of NYC Health + Hospitals, the nation’s largest public health system. For a list of MetroPlusHealth locations and hours of operations, visit metroplus.org/metroplus-near-you. Research Methodology MetroPlusHealth’s 2021 Survey of Behavioral Health was conducted in September 2021 and consists of two distinct studies with the Geo-CARAVAN survey conducted by ENGINE INSIGHTS. The NYC survey was among New York City DMA residents, 18 years of age and older. The general population survey was among a sample of 1,011 adults 18 years of age and older. The online omnibus study is conducted three times a week among a demographically representative U.S. sample of 1,000 adults 18 years of age and older. About ENGINE INSIGHTS ENGINE INSIGHTS is a collaborative and consultative research partner to hundreds of organizations around the globe. We possess a wide variety of resources, tools and technologies to collect and analyze information for our clients. As a member of the Insights Association and ESOMAR (the European Society for Opinion and Marketing Research), ENGINE INSIGHTS adheres to industry ethics and best practices, including maintaining the anonymity of our respondents. Contact Details Divendra Jaffar 212-908-3380 +1 646-952-3243 jaffadi@metroplus.org
December 06, 2021 11:23 AM Eastern Standard Time
 News Release
News Release